On September 24 of this year, the Food and Drug Administration (FDA) issued a final rule to update the Mammography Quality Standards Act of 1992 (MQSA). When a woman has a mammogram, the FDA now requires specific language in her report to inform her about her breast density measurement and the increased risks that come with having denser breasts. It also requires she be informed that having dense breasts makes it more difficult to find breast cancer on mammograms and that she may benefit from additional forms of screening.
Up to 40% of women have dense breasts, but many do not know they have them. Over the past two decades, 38 states have passed laws requiring some form of breast density notification. The new federal rule was issued to fill the gaps among the states where no notification laws existed, and to fix inconsistencies in states where such notifications were required.
According to the FDA, the MQSA was established to improve the delivery of mammography services, enable more informed decision making by patients and providers, improve outcomes, modernize guidelines and more. By standardizing the information that women must receive, the FDA wants all women to understand the risks that breast density poses and what they can do about it.
The rationale behind this historic decision is twofold. First, the increased cancer risk experienced by women with dense breasts. There is now clinical evidence that women with dense breasts are up to 6X more likely to develop breast cancer.1 According to Komen, dense breasts have higher levels of certain molecules that contribute to inflammation, and tissue stiffness and remodeling, which can contribute to tumor formation.
Second, the unique challenge of finding cancer in a dense breast using a traditional 2D mammogram. Mammograms are basically x-rays of the breast. Fibroglandular tissue, which women with dense breasts have more of, appears white on an x-ray image. But so does cancer. This makes it especially difficult for even the most highly trained radiologist to spot cancer.
In recent years, an innovation in mammographic screening called digital breast tomosynthesis (also known as 3D mammography) has been shown to improve the detection rate for women with dense breasts. Additionally, supplemental screening tests such as ultrasound and breast MRI have been used to improve detection rates for these women.
The FDA advises women to talk with their healthcare providers if their latest mammogram reveals they have dense breasts. However, not all doctors and practitioners are fully aware of the risks that dense breasts pose, as this issue has only recently come to light. (The first state to pass a breast density notification law was Connecticut in 2009). Therefore, it is important that a woman conduct her own research and learn what she can do to ensure she receives the most appropriate form of screening.
Are You Dense? was one of the first non-profit organizations to bring this issue to light and has been a driving force behind many of the state and federal mandates for breast density notification. Their website, www.areyoudense.org is a good starting point for women who want to know more about breast density and what it means for them.
BREAST DENSITY IS MEASURED DURING YOUR ANNUAL SCREENING MAMMOGRAM
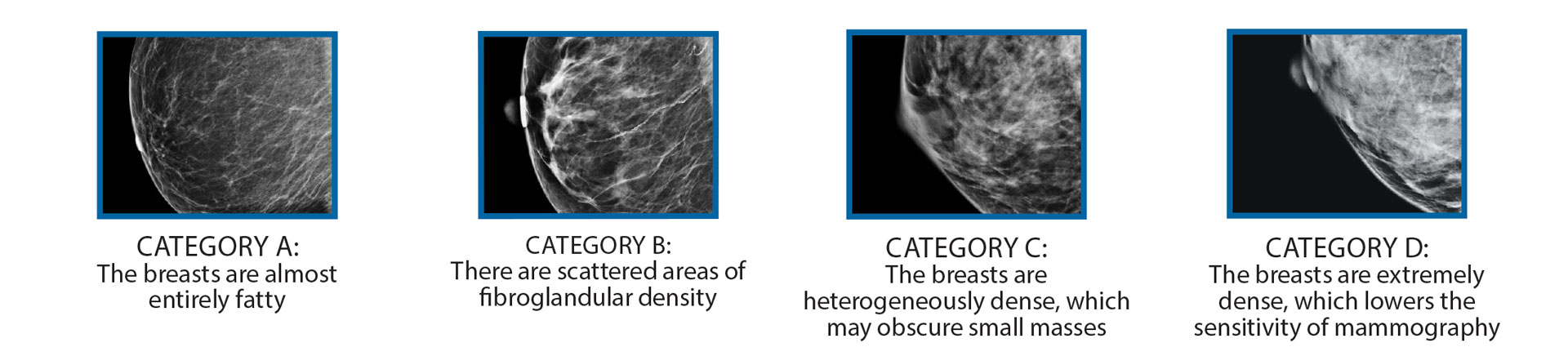
All mammography reports from RadX Imaging Partners contain a classification of breast density based on the Breast Imaging Reporting and Data System (BI-RADS) breast composition categories:
- Margaret T. Mandelson et al Breast Density as a Predictor of Mammographic Detection: Comparison of Interval- and Screen-Detected Cancers JNCI: Journal of the National Cancer Institute, Volume 92, Issue 13, 5 July 2000, Pages 1081–1087, “https://doi.org/10.1093/jnci/92.13.1081”